Which Property of Water Causes the Curved Surface
A hydrogen bond can form between a hydrogen atom of one water molecules and which atom of another water molecule. Oxygen and hydrogen sides being slightly positive.
Cohesion And Adhesion Of Water Article Khan Academy
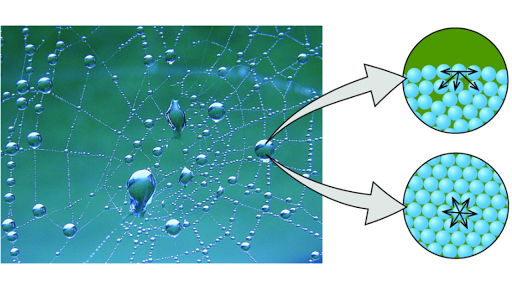
Is a combination force of attraction among water molecules and all the surrounding materials.

. Up to 24 cash back polarity. Mercury does not adhere to glass so mercury in a cylinder will curve the other way - convex. As you may have noticed when water is in such a thin glass tube it does not have a flat surface at the top.
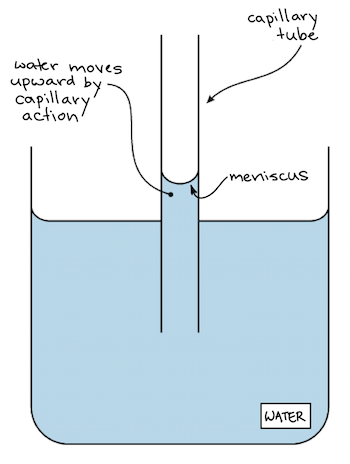
The force pulling the water up is called capillary action. New westminsterbc canda. The water molecules are more strongly attracted to the glass than they are to other water molecules because glass molecules.
If the adhesive force is greater than the cohesive force the molecules at the sides will try to hug the container which causes the curved meniscus at the surface. The bonds that hold the hydrogen to the oxygen are. Water molecules are polar with the.
The tendency for water molecules form weak bonds and they stick to each other. A meniscus is the curved surface at the top of a column of liquid. The tendency of water to stick to other substances.
Small insects such as water striders can walk on water by taking advantage of this surface tension. Temperature of the surface ocean water varies seasonally with warmer temperatures recorded in summer and colder temperatures in winter. The force of adhesion between water and glass molecules is greater than the force of cohesion between two water moleculesThis causes the surface of the water to be curved.
This occurs with water and a glass tube. In a graduated cylinder you would read the water level from the bottom of the curvature and the mercury level from the top. This force causes molecules at the surface to be held more tightly together forming a kind of skin at the waters surface.
Learn vocabulary terms and more with flashcards games and other study tools. Heat capacity Adhesion property of. The water molecule is neutral.
Instead the top is curved. Start studying Properties of Water. When we study ocean temperatures we usually consider the annual average.
Water tends to attract and be attracted to other polar molecules. Oxygen side being slightly positive and the hydrogen side being slightly negative. This curved surface is.
Adhesion is a water property that allows water molecules to adhere to each other. When the slope occurs the water surface becomes curved as shown in the figure. A concave meniscus which is what you normally will see occurs when the molecules of the liquid are attracted to those of the container.
This attraction between water molecules and other polar molecules is called adhesion. If the polar water molecules can stick to a surface adhesion wins and you will get a concave hollow surface. The narrower the tube the lighter the column of water for a given contact area with the tube and the greater the.
The unequal sharing of electrons gives the water molecule a slight negative charge near its hydrogen atoms and a slight positive charge near its oxygen atom. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the container as with mercury and glass. Powered by adhesion which causes water.
This can occur thanks to its polarity. The molecule has two poles at which the it is colder than other regions of the molecule. Up to 24 cash back the surface of the water however the attractive force of other water molecules pulls only downward and sideways.
Which property of water causes the curved surface shown in Figure 2-1. The Polar molecule makes one end positive and the other end negative. On this basis surface ocean temperatures range from about 28 o C in the tropical western Pacific to 3 o C off the coast of Antarctica.
This upward motion against gravity known as capillary action depends on the attraction between water molecules and the glass walls of the tube adhesion as well as on interactions between water molecules cohesion. Which property of water causes the curved surface shown in the figure below. In a science class this liquid is usually water or some sort of aqueous solution and the column is usually a graduated cylinder or a pipet.
The force of adhesion between water and glass molecules is greater than the force of cohesion between two water moleculesThis causes the surface of the water to be curved.
Cohesion And Adhesion Of Water Article Khan Academy

Liquid Properties Boundless Chemistry

Which Property Of Water Causes The Curved Surface Shown In The Figure Below A Heat Capacity B Brainly Com

The Properties Of Water The Chemical Formula For Water Is H 2 O This Mean That Each Molecule Of Water Is Made From Two Atoms Of Hydrogen And One Atom Ppt Download
Comments
Post a Comment